


How Strip Packaging and Smart Line Integration Are Shaping the Future of Pharma?
In the pharmaceutical industry, primary packaging serves as the first line of defense for drug stability and is a critical link between manufacturing processes and patient safety. As two mainstream high-barrier, unit-dose solutions, the choice between strip packaging and Alu-Alu blister packaging directly impacts a drug's shelf life, production costs, and patient compliance. With increasingly stringent global regulatory requirements, the efficient integration of primary packaging with downstream automatic cartoners and carded packaging machines has become central to building compliant, agile, and modern pharmaceutical production lines.
This article provides an in-depth analysis of both packaging technologies, compares their characteristics, and offers a comprehensive selection framework for professional readers targeting the European and American markets.
Strip packaging is a continuous process where tablets or capsules are precisely sealed between two rolls of flexible laminate film, forming individual flat units. Its core technology lies in the precise thermal sealing control of multilayer co-extruded high-barrier films.
Materials Science: High-performance strip packaging films typically have a 3 to 5-layer composite structure. A common configuration includes: an outer layer (print layer, e.g., Polyester PET), a core barrier layer (20-40 micron aluminum foil, providing an absolute barrier against moisture, light, and oxygen), and an inner heat-seal layer (e.g., Cast Polypropylene CPP or special polyethylene). The water vapor transmission rate (WVTR) of the aluminum foil can be below 0.01 g/m²/day, with an oxygen transmission rate (OTR) near zero.
Process and Line Integration: Strip packaging machines achieve highly synchronized operations: bottom web unwinding, vision-guided positioning, precise measured filling, top web application, thermal sealing (via precision heated rollers or platens), and finally die-cutting or cold punching to form individual strips. This design allows for direct inline integration with downstream high-speed automatic cartoners. Strip products can be fed directly into the cartoning process via conveyors or robotic arms, achieving speeds exceeding 400 cartons per minute.
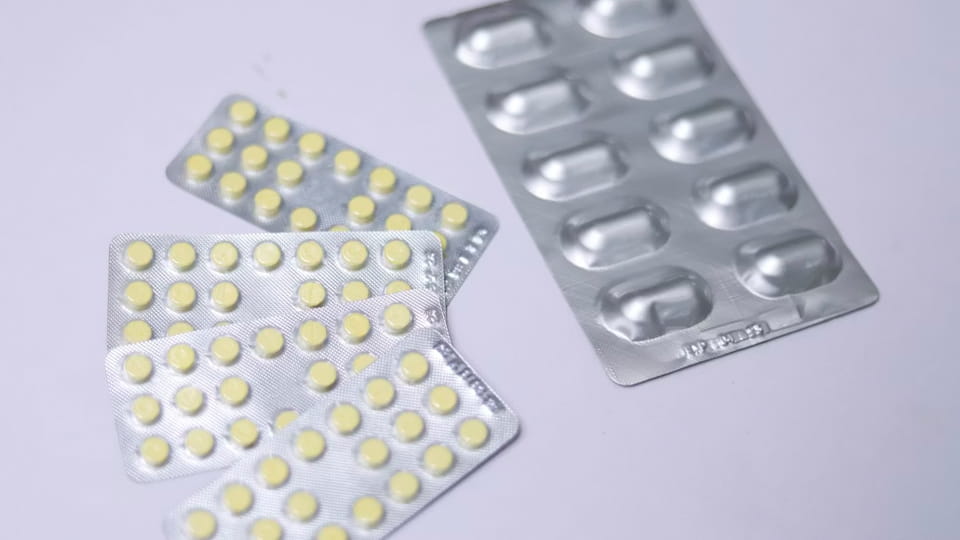

Alu-Alu blister packaging, also known as cold-form blister packaging, is a rigid, all-metal barrier packaging form. It does not use traditional PVC or PET plastic bases. Instead, it employs cold-forming technology to stamp specially engineered aluminum-based composite foil at room temperature, creating cavities that hold the tablets.
Definition and Structure: Its complete structure consists of two parts: the Cold-Formed Aluminum (CFA) base web and the lidding foil. The CFA base is typically a composite of "Polyamide (Nylon)/Aluminum foil/Polyvinyl Chloride (PVC) or Polypropylene," where the aluminum foil (often 45 microns) provides the main barrier, nylon offers toughness, and the inner PVC facilitates cold forming. The lidding foil is responsible for sealing and is often printed with product information.
How It Works: On a dedicated cold-form blister machine, the CFA base web is stamped into cavities by a steel mold under high pressure—a process conducted at room temperature, avoiding stress changes in the material caused by heating. After tablet filling, the lidding foil is heat-sealed onto the flange of the cavity. Due to its all-metal, sealed structure, it offers the highest commercially available level of protection, completely blocking light, moisture, and oxygen.
Alu-Alu blister packaging is the preferred choice for high-value, highly sensitive drugs due to a series of irreplaceable advantages:
Superior Barrier Properties: Complete barrier against water vapor, oxygen, and UV light, providing ultimate protection for APIs prone to hydrolysis or oxidation, significantly extending drug shelf life.
Excellent Physical and Chemical Stability: The rigid aluminum structure offers outstanding resistance to crushing and puncture, effectively protecting brittle tablets or capsules from damage during transportation. Aluminum is also chemically inert, preventing interaction with the drug.
Enhanced Safety and Compliance: Facilitates robust Child-Resistant (CR) opening mechanisms and clear Tamper-Evident designs. Its opacity also protects patient privacy.
Patient-Friendly: Enables easy "push-through" removal of tablets without tearing, which is particularly beneficial for elderly patients or those with reduced dexterity, improving medication adherence.
Brand and Quality Perception: In European and American markets, Alu-Alu blister packaging is often associated with innovator drugs, patented pharmaceuticals, and high-quality generics, reinforcing a brand's professional and premium image.
Efficient integration with downstream equipment is key to ensuring overall output and meeting regulatory and market requirements after primary packaging.
Automatic Cartoner: The modern servo-driven cartoner is the hub of the packaging line. It must not only perform at high speeds (up to 500 cartons/minute) the tasks of erecting cartons, inserting blisters/strips, inserting leaflets, and closing cartons but also integrate serialization and aggregation functionalities. To comply with regulations like the US Drug Supply Chain Security Act (DSCSA) and the EU Falsified Medicines Directive (FMD), cartoners must be equipped with vision systems to verify product identification codes on primary packaging and print unique serial numbers, batch numbers, and expiration dates on each saleable unit carton, while establishing aggregation links with higher-level shipping cases.
Carded Packaging Machine: Primarily used for OTC drugs and supplements, this machine heat-seals or glues single or multiple blister packs onto pre-printed card stock. It integrates functions like precise positioning, heat-seal control, and die-cutting. The finished card serves as an important marketing and patient education tool, clearly displaying branding, usage instructions, compliance symbols, and tamper-evidence features.
Choosing between strip packaging and Alu-Alu blister packaging requires a systematic evaluation across multiple dimensions. Here is a key comparative analysis:
Protection Performance Comparison: When aluminum foil is used as the barrier layer, both can provide extremely high moisture and oxygen barrier properties. However, the rigid, all-metal structure of Alu-Alu blisters is absolutely superior in physical protection (crush and shock resistance), making it especially suitable for dosage forms sensitive to mechanical damage, such as friable tablets, matrix tablets, or certain capsules. The flexibility of strip packaging may subject contents to pressure under extreme force.
Production Cost and Efficiency Comparison: Strip packaging typically holds a significant advantage in material cost and production speed. It uses thinner, lighter composite films, and its production process is highly continuous with relatively smaller equipment footprint and lower energy consumption. The cold-form aluminum material for Alu-Alu blisters is costly, the cold-forming process is generally slower than the thermal sealing process for strips, and the equipment investment is higher.
Patient Use and Market Adaptability Comparison: The "push-through" opening of Alu-Alu blisters is more convenient, offering a better user experience. Strip packages require tearing, which, while designable to be child-resistant, might be slightly less convenient for some users. In terms of market positioning, Alu-Alu blisters are the standard choice for high-end prescription and specialty drugs; strip packaging is widely used for generics, hospital dispensing, high-volume packaging, and cost-sensitive markets.
Line Integration and Flexibility Comparison: Strip packaging reels facilitate easier high-speed, automated cartoning and case packing. Rigid Alu-Alu blister cards are also efficient for robotic picking and positioning but may require more consideration for buffering and orientation in line layout. For scenarios requiring a high degree of customization (e.g., clinical trial supplies, multi-drug combination packs), strip packaging allows for flexible changes simply by altering printing and cutting programs, whereas Alu-Alu blisters require expensive mold changes.
Sustainability Comparison: Both face challenges in recycling composite materials. Strip packaging has a relatively lower carbon footprint due to less material used. The aluminum in Alu-Alu blisters is infinitely recyclable, but separating the composite layers is technically difficult. The industry trend is toward developing single-material, high-barrier plastics, but they have not yet fully replaced existing solutions.

The choice is not binary but a balance based on specific product and strategy.
Prioritize Alu-Alu Blister Packaging, if:
Your drug's active ingredient is extremely sensitive to moisture, oxygen, or light.
The drug is high-value (e.g., biologics, oncology drugs), where packaging cost is a relatively small proportion, and the return from top-tier protection in market trust and reduced spoilage is significant.
The target market (e.g., mainstream prescription markets in the EU/US) has a strong preference or regulatory guidelines recommend rigid blister packaging.
The dosage form is fragile and requires a rigid container for physical protection.
Brand building requires conveying a clear message of high quality and safety.
Prioritize Strip Packaging, if:
Cost control is the primary consideration, and you need the most cost-effective high-barrier solution for generics or bulk pharmaceuticals.
The product requires extremely long continuous production runs and maximum line speed.
The packaging design needs high flexibility, such as variable dosing, multi-drug combinations, or frequent product line changeovers.
The supply chain emphasizes compact storage and transportation, where the lightweight, flexible nature of strip packaging can significantly reduce logistics costs.
The drug has low physical protection requirements but high barrier needs.
In many cases, the final decision should be based on a detailed Total Cost of Ownership (TCO) analysis, accounting not only for packaging material costs but also equipment investment, operational efficiency, benefits from extended shelf life, and potential brand premium.
Q: Is Alu-Alu blister packaging always more moisture-proof than strip packaging?
A: Not necessarily. The key to moisture barrier performance for both lies in whether they contain a continuous, defect-free aluminum foil layer. A well-designed strip package using an aluminum-aluminum composite film can achieve a WVTR on par with top-tier Alu-Alu blisters (both below 0.1 g/m²/day). The main differences lie in physical strength and form.
Q: What are the most important compliance considerations when choosing packaging for products targeting the EU/US markets?
A: Beyond the packaging materials themselves needing to comply with relevant material standards (e.g., USP, EP), the most critical aspect is serialization and traceability. The packaging format you choose (especially its scannable surface) and the integrated cartoner must be capable of efficiently and reliably applying, verifying, and aggregating unique serial numbers at the item level to meet DSCSA and FMD regulations.
Q: Can strip packaging be made Child-Resistant (CR)?
A: Yes. Through the use of special film materials and heat-seal patterns, strip packaging can be designed to require a specific technique and force to tear open, thereby complying with child-resistant packaging standards (e.g., US 16 CFR 1700). However, this must be balanced with ease of opening for patients, especially the elderly.
Q: In a production line, which packaging format typically has a shorter changeover time?
A: Strip packaging machines are usually faster to change over. Switching products mainly involves changing film reels and adjusting sealing parameters, which can be done quickly using servo motors and stored recipes. Changing the blister size or shape on an Alu-Alu blister machine often requires replacing expensive forming and cutting dies, which is more time-consuming.
Q: What are the improvement trends for these two packaging types regarding sustainability?
A: The industry is moving in two main directions: First, developing recyclable, single-material polymer high-barrier films (e.g., transparent high-barrier films based on PP or PE) to replace hard-to-recycle aluminum-plastic laminates. Second, optimizing design to reduce material usage (e.g., using thinner aluminum foil, minimizing blister flange width). Additionally, using renewable or recycled source paperboard for outer cartons and cards is a significant environmental initiative.
Key Literature & Technical References
International Journal of Pharmaceutics: "Water Vapor Permeability of Pharmaceutical Packaging Materials".
USP General Chapters: <671> "Containers—Performance Testing".
European Pharmacopoeia: "Materials for Containers and Containers".
Parenteral Drug Association (PDA) Technical Report: "Serialization in the Pharmaceutical Supply Chain".
AAPS PharmSciTech: "A Comparative Study of Cold-Form Blister and Strip Packaging for Moisture-Sensitive Drugs".

 Jinhai Plaza, No. 21, Jihua 5th Road, Chancheng District, Foshan City, Guangdong Province, China
Jinhai Plaza, No. 21, Jihua 5th Road, Chancheng District, Foshan City, Guangdong Province, China  +0757 82252350
+0757 82252350  +8618613054883
+8618613054883  manager@gdboanmachine.com
manager@gdboanmachine.com 

